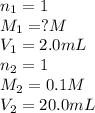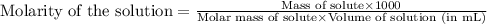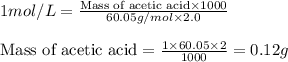Chemistry, 25.02.2020 05:12, rosetoheart2
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titrate it to its endpoint with 20.0 mL NaOH (0.1 M). What mass of acetic acid was dissolved in the 2.0 mL of solution used?

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
Do you know the correct answer?
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titra...
Questions in other subjects:











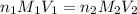
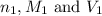 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 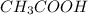
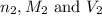 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.