
The proposed mechanism for a reaction is 1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW 2. I-(aq) + HClO(aq) => HIO(aq) + Cl-(aq) FAST 3. OH-(aq) + HIO(aq) => H2O(l) + IO-(aq) FAST Which of the following would be a rate law for the reaction? A. rate = k[ClO-][H2O] B. rate = k[I-][HClO] C. rate = k[OH-][HIO] D. rate = k[ClO-][H2O][I -] E. rate = k[ClO-][H2O][I-][OH-]

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1


Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
Do you know the correct answer?
The proposed mechanism for a reaction is 1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW 2. I-(aq...
Questions in other subjects:


Computers and Technology, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30


History, 10.05.2021 17:30

Social Studies, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30

English, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30


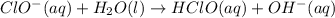
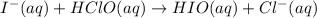
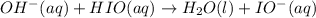
![R=k[ClO^-][H_2O]](/tpl/images/0522/8081/8de77.png)




