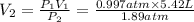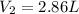Chemistry, 25.02.2020 04:02, madisongibson714
Calculate the volume the gas will occupy if the pressure is increased to 1.89 atmatm while the temperature is held constant. Express the answer in liters to three significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, Islandgirl67
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b. zinc7.14,c. copper 8.92,d. lead 11.34
Answers: 2


Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
Calculate the volume the gas will occupy if the pressure is increased to 1.89 atmatm while the tempe...
Questions in other subjects:

Computers and Technology, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

History, 14.12.2020 14:00


Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00


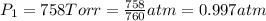
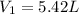

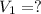
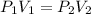 ( at constant temperature)
( at constant temperature)