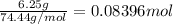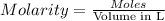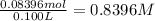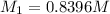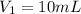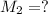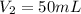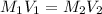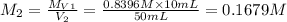Chemistry, 25.02.2020 04:01, swelch2010
. A student transferred a 10-mL aliquot of a stock sodium hypochlorite solution into a 50-mL volumetric flask using a volumetric pipet. The solution was then diluted to the mark with distilled water. Assuming that the concentration of a stock sodium hypochlorite is 6.25% (w/v), calculate the molarity of the diluted sodium hypochlorite solution. Molar mass of sodium hypochlorite

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
Do you know the correct answer?
. A student transferred a 10-mL aliquot of a stock sodium hypochlorite solution into a 50-mL volumet...
Questions in other subjects:

Spanish, 14.05.2021 21:50

Mathematics, 14.05.2021 21:50