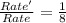Chemistry, 25.02.2020 04:05, alialbinn6969
The gas phase reaction between NO and O2 is second order with respect to NO and first order with respect to 02, what would happen to the reaction rate if the concentrations of both reactants were halved with everything else held constant?
a. It would decrease by a factor of 8 It would remain unchanged.
b. It would decrease by a factor of 16.
c. It would decrease by a factor of 4.
d. It would decrease by a factor of 2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
Do you know the correct answer?
The gas phase reaction between NO and O2 is second order with respect to NO and first order with res...
Questions in other subjects:

English, 04.03.2020 04:16


Chemistry, 04.03.2020 04:16

Mathematics, 04.03.2020 04:17

Mathematics, 04.03.2020 04:17

History, 04.03.2020 04:17


Mathematics, 04.03.2020 04:17


English, 04.03.2020 04:17


![Rate=k[NO]^2[O_2]^1](/tpl/images/0522/7517/1df0d.png) (1)
(1)![Rate'=k{\frac{[NO]}{2}^2}{\frac{[O_2]}{2}^1}](/tpl/images/0522/7517/19c10.png) (2)
(2)![\frac{Rate'}{Rate}=\frac{k\frac{[NO]}{2}^2\times \frac{([O_2]}{2})^1}{k[NO]^2[O_2]^1}](/tpl/images/0522/7517/9c613.png)