Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
Do you know the correct answer?
A sample containing 3.25 moles of He is mixed with 2.25 moles of Ne and 1.75 moles of Ar. All compon...
Questions in other subjects:


Mathematics, 20.03.2021 01:10

Mathematics, 20.03.2021 01:10







Mathematics, 20.03.2021 01:10


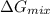 is, -22.5 kJ
is, -22.5 kJ![\Delta G_{mix}=nRT\times [X_{He}\ln (X_{He})+X_{Ne}\ln (X_{Ne})+X_{Ar}\ln (X_{Ar})]](/tpl/images/0522/4111/f4a32.png)
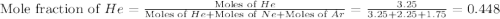
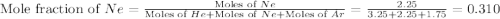
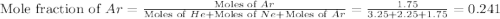
![\Delta G_{mix}=(7.25mol)\times (8.314J/mol.K)\times (350K)\times [0.448\ln (0.448)+0.310\ln (0.310)+0.241\ln (0.241)]](/tpl/images/0522/4111/274c3.png)