
Chemistry, 25.02.2020 01:47, kitttimothy55
Consider the following reaction at equilibrium: 2 NH3(g) ⇄ N2(g) + 3 H2(g) What does Le Chatelier's principle predict will happen when adding N2 (g) to the system at equilibrium?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
Do you know the correct answer?
Consider the following reaction at equilibrium: 2 NH3(g) ⇄ N2(g) + 3 H2(g) What does Le Chatelier's...
Questions in other subjects:



Health, 06.12.2020 21:20


World Languages, 06.12.2020 21:20


Mathematics, 06.12.2020 21:20


English, 06.12.2020 21:30

Physics, 06.12.2020 21:30


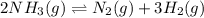
 then the concentration of
then the concentration of 



