Chemistry, 25.02.2020 00:50, SucMaDongShan
For a particular reaction at 298 K, the equilibrium constant is equal to 581. Determine ΔG° in kJ/mol for the reaction. Do not include units. Report your answer to 3 significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 07:40, Aaron5795
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 08:00, johngayden46
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
Do you know the correct answer?
For a particular reaction at 298 K, the equilibrium constant is equal to 581. Determine ΔG° in kJ/mo...
Questions in other subjects:



Health, 18.02.2021 22:00







Mathematics, 18.02.2021 22:00


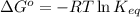
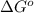 = Standard Gibbs free energy = ?
= Standard Gibbs free energy = ?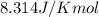
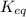 = equilibrium constant = 581
= equilibrium constant = 581