

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
Do you know the correct answer?
Nitroglycerine decomposes violently according to the chemical equation below. What mass of carbon di...
Questions in other subjects:


English, 24.08.2020 20:01

Mathematics, 24.08.2020 20:01

Mathematics, 24.08.2020 20:01

Physics, 24.08.2020 20:01


History, 24.08.2020 20:01


History, 24.08.2020 20:01

English, 24.08.2020 20:01


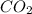 produced is, 7.74 grams
produced is, 7.74 grams = 13.3 g
= 13.3 g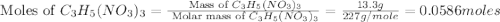
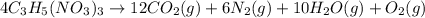
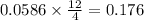 moles of
moles of 





