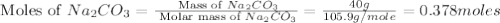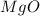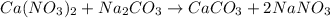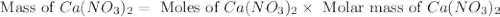Chemistry, 25.02.2020 00:41, melissareid65
Redo the experiment by clicking on Reset Experiment. Add Ca(NO3)2 to 40 g of Na2CO3 and determine at what point the masses of the two reactants react "evenly." That is, how many grams of Ca(NO3)2 must be added to just consume the 40 g Na2CO3 initially available?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, lileljusto2829
Which is the most likely way an automotive engineer would use chemistry
Answers: 1

Chemistry, 23.06.2019 00:30, StayPuftMarshadowMan
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
Do you know the correct answer?
Redo the experiment by clicking on Reset Experiment. Add Ca(NO3)2 to 40 g of Na2CO3 and determine at...
Questions in other subjects:



English, 13.05.2021 20:20

Geography, 13.05.2021 20:20

Mathematics, 13.05.2021 20:20

Mathematics, 13.05.2021 20:20





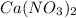 added must be, 61.9 grams
added must be, 61.9 grams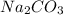 = 40 g
= 40 g