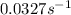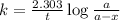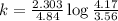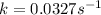Chemistry, 24.02.2020 23:41, davisbrittany5784
Consider the following first-order reaction: A → B. The concentration of A at the start of the reaction is 4.17 M and after 4.84 s is 3.56 M. (a) Using the integrated rate law for a first-order reaction, calculate the value of the rate constant.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 09:00, rebeccathecatt
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
Do you know the correct answer?
Consider the following first-order reaction: A → B. The concentration of A at the start of the react...
Questions in other subjects:


Mathematics, 26.01.2021 20:00


Mathematics, 26.01.2021 20:00




Social Studies, 26.01.2021 20:00


Mathematics, 26.01.2021 20:00