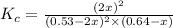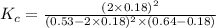Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g) ⇌2NOCl(g) A reaction mixture at a certain temperature initially contains only [NO]=[NO]= 0.53 MM and [Cl2]=[Cl2]= 0.64 MM. After the reaction comes to equilibrium, the concentration of NOClNOCl is 0.36 MM. Part A Find the value of the equilibrium constant (Kc)(Kc) at this temperature.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
Do you know the correct answer?
Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g)...
Questions in other subjects:

Social Studies, 18.09.2019 14:00


Social Studies, 18.09.2019 14:00

Mathematics, 18.09.2019 14:00


English, 18.09.2019 14:00

Mathematics, 18.09.2019 14:00


Health, 18.09.2019 14:00

Chemistry, 18.09.2019 14:00


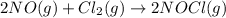
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0521/6978/56950.png)