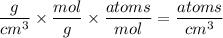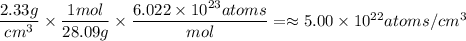Chemistry, 24.02.2020 19:01, davgre1271
Compute the atomic density (the number of atoms per cm3 ... rather than the mass density g/cm3) for a perfect crystal of silicon at room temperature, given that the room temperature density and atomic weight of silicon are 2.33 g/cm3 and 28.09 g/mol, respectively.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3


Chemistry, 23.06.2019 07:30, 22emilyl530
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
Do you know the correct answer?
Compute the atomic density (the number of atoms per cm3 ... rather than the mass density g/cm3) for...
Questions in other subjects:





Mathematics, 22.10.2019 03:00

Mathematics, 22.10.2019 03:00