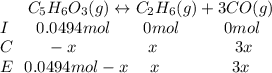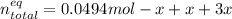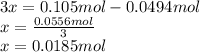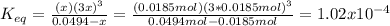Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g sample of pure C5H6O3(g) was placed in an evacuated 2.50 L flask and heated to 200.ºC. At equilibrium, the pressure in the flask was 1.63 atm. Calculate K for this reaction.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
Do you know the correct answer?
Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g...
Questions in other subjects:

Health, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20


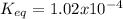
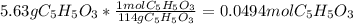
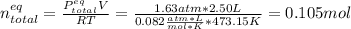
 ", which yields to:
", which yields to: