Chemistry, 24.02.2020 18:28, ninja12302
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 320.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
Do you know the correct answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions in other subjects:

English, 17.12.2020 20:20



Mathematics, 17.12.2020 20:20

Mathematics, 17.12.2020 20:20


Business, 17.12.2020 20:20

Biology, 17.12.2020 20:20


Spanish, 17.12.2020 20:20


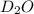 needed is 0.96 grams
needed is 0.96 grams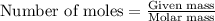 .....(1)
.....(1) :
: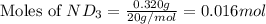

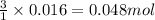 of
of