Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3


Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
At 380 0C, the equilibrium concentrations are [CH3OH] = 0.20 M, [CO] = 0.35 M, and [H2] = 2.1 M for...
Questions in other subjects:


Mathematics, 17.12.2019 11:31


Biology, 17.12.2019 11:31



English, 17.12.2019 11:31



Social Studies, 17.12.2019 11:31


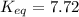
![K_{eq}=\frac{[CO]_{eq}[H_2]^2_{eq}}{[CH_3OH]_{eq}}](/tpl/images/0521/2714/cf196.png)