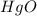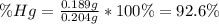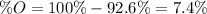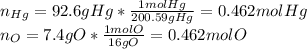Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 03:10, mani1682
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
Do you know the correct answer?
An oxide of mercury will thermally decompose when heated. A 0.204 gram sample of the mercury oxide i...
Questions in other subjects:

Biology, 19.03.2021 19:20

Mathematics, 19.03.2021 19:20

History, 19.03.2021 19:20




Mathematics, 19.03.2021 19:20

Mathematics, 19.03.2021 19:20


Mathematics, 19.03.2021 19:20