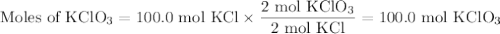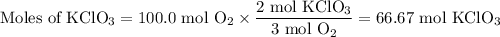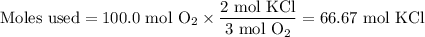Chemistry, 24.02.2020 07:21, DASASDAEDWEDA
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of KCl and 100.0 moles of O2...
what is the limiting reactant? (TYPE EITHER KCl or O2)
what is the excess reactant? (TYPE EITHER KCl or O2)
how many moles of the excess reactant will be left over? moles (TYPE just the number to the correct amount of significant figures)

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
Do you know the correct answer?
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of...
Questions in other subjects:

Mathematics, 12.10.2019 18:30

History, 12.10.2019 18:30




Mathematics, 12.10.2019 18:30


English, 12.10.2019 18:30


Mathematics, 12.10.2019 18:30