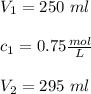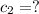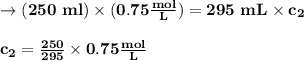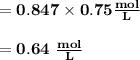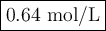Chemistry, 23.02.2020 04:47, brainlord4209
Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 solution, what will the
molarity of the diluted solution be?
(0.75 M)(250 ml) = M2 (295 mL)
M2 = (0.75 M) (250 mL) = 0.64 M
(295 mL)
Where did the 295ml came from

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
Do you know the correct answer?
Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 soluti...
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 soluti...
Questions in other subjects:




Mathematics, 09.02.2021 05:50

Mathematics, 09.02.2021 05:50

Biology, 09.02.2021 05:50




Mathematics, 09.02.2021 05:50