Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
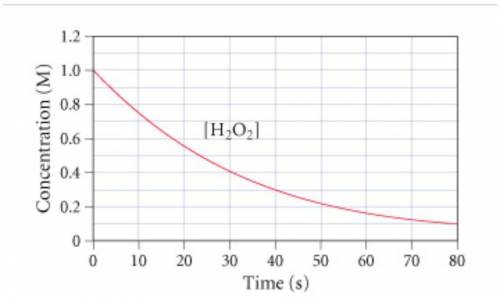
The graph (Figure 1...

Chemistry, 22.02.2020 20:03, beanokelley
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L , what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3

Chemistry, 23.06.2019 22:40, bicaplinger7766
For the following reaction, 3.70 grams of oxygen gas are mixed with excess carbon monoxide . the reaction yields 8.25 grams of carbon dioxide . carbon monoxide(g) + oxygen(g) carbon dioxide(g) what is the ideal yield of carbon dioxide? grams what is the percent yield for this reaction? %
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Geography, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10

Computers and Technology, 02.03.2021 18:10



Social Studies, 02.03.2021 18:10









