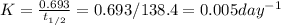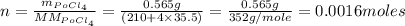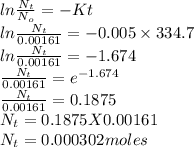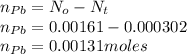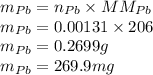Chemistry, 22.02.2020 06:17, antbanks3050
Polonium-210, 210Po, decays to lead-206, 206Pb, by alpha emission according to the equation
210 206 4
84 Po > 82Pb + 2 He
If the half-life, t1/2, of 210Po is 138.4 days, calculate the mass of 206Pb that can be produced from a 565.0-mg sample of polonium(IV) chloride, PoCl4, that is left untouched for 334.7 days. Assume that the only polonium isotope present in the sample is the 210Po isotope. The isotopic molar masses of 210Po is 209.98 g/mol and 206Pb is 205.97 g/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monithebtslover01
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1


Chemistry, 21.06.2019 22:30, 20heldmadison
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
Do you know the correct answer?
Polonium-210, 210Po, decays to lead-206, 206Pb, by alpha emission according to the equation
Questions in other subjects:



Social Studies, 20.09.2020 21:01




English, 20.09.2020 21:01



Mathematics, 20.09.2020 21:01