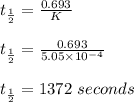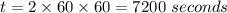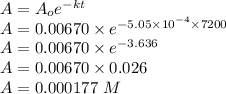Chemistry, 22.02.2020 04:59, francisebell2p698f2
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene gas. At a certain temperature, the rate constant for this reaction is 5.05 × 10 − 4 s − 1 . Calculate the half-life of cyclopropane at this temperature. t 1 / 2 = s Given an initial cyclopropane concentration of 0.00670 M , calculate the concentration of cyclopropane that remains after 2.00 hours. concentration.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3


Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene g...
Questions in other subjects:


Arts, 09.04.2021 17:00


Business, 09.04.2021 17:00


Mathematics, 09.04.2021 17:00


Mathematics, 09.04.2021 17:00

Social Studies, 09.04.2021 17:00

Chemistry, 09.04.2021 17:00


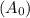 of was
of was 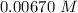 .
. is
is 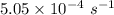
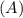 after 2 hours.
after 2 hours.