Chemistry, 22.02.2020 02:58, borgesalfonso12
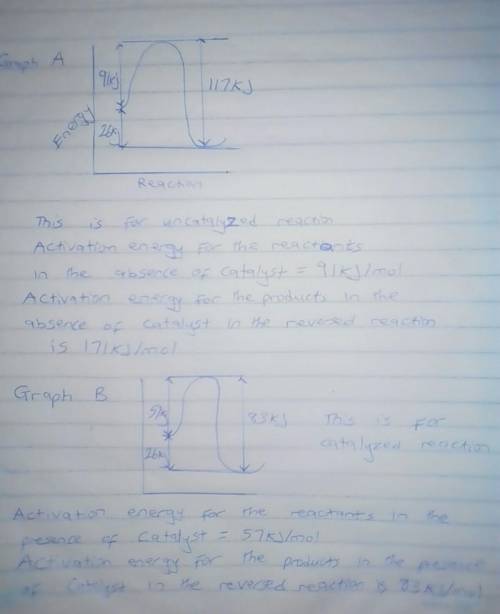
A catalyst decreases the activation energy of a particular exothermic reaction by 34 kJ/mol, to 57 kJ/mol. Assuming that the mechanism has only one step, and that the products are 26 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions. What is the activation energy for the uncatalyzed reverse reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
Do you know the correct answer?
A catalyst decreases the activation energy of a particular exothermic reaction by 34 kJ/mol, to 57 k...
Questions in other subjects:









Social Studies, 18.10.2021 22:40