

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, saby30
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1



Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
Do you know the correct answer?
Order the following compounds according to their expected lattice energies: AlBr3,MgBr2, LiBr, and C...
Questions in other subjects:

Mathematics, 03.04.2020 01:48

Mathematics, 03.04.2020 01:48


Mathematics, 03.04.2020 01:48

Chemistry, 03.04.2020 01:48

History, 03.04.2020 01:48




Mathematics, 03.04.2020 01:48


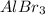 > CaO >
> CaO > 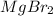 > LiBr
> LiBr



