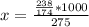Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate the molarity of a solution prepared by dissolving 238 g of K2HPO4 in enough water to produce 275 mL of solution. A) 0.732 M B) 0.865 M. C) 2.66 M D) 4.97 M E) none of the above

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1


Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Do you know the correct answer?
Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate th...
Questions in other subjects:





Chemistry, 24.07.2019 12:00

Mathematics, 24.07.2019 12:00



Mathematics, 24.07.2019 12:00

Mathematics, 24.07.2019 12:00


 moles.
moles.