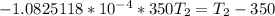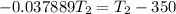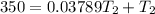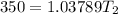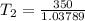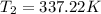Chemistry, 22.02.2020 00:13, jak000067oyyfia
The normal boiling point of liquid ethyl acetate is 350 K. Assuming that its molar heat of vaporization is constant at 34.4 kJ/mol, the boiling point of CH3COOC2H5 when the external pressure is 0.639 atm is K.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
Do you know the correct answer?
The normal boiling point of liquid ethyl acetate is 350 K. Assuming that its molar heat of vaporizat...
Questions in other subjects:


Mathematics, 04.02.2020 04:56


Social Studies, 04.02.2020 04:56

Mathematics, 04.02.2020 04:56

Computers and Technology, 04.02.2020 04:56


Chemistry, 04.02.2020 04:56



![In\frac{P_2}{P_1}=\frac{\delta H_{vap}}{R}[\frac{T_2-T_1}{T_2T_1}]](/tpl/images/0519/8584/04ef0.png)
![In\frac{0.639}{1}=(\frac{34.4*10^3J/mol}{8.314 J K^{-1}mol^{-1}})[\frac{T_2-350}{350T_2}]](/tpl/images/0519/8584/b0f94.png)
![In({0.639})=(\frac{34.4*10^3}{8.314K^{-1}})[\frac{T_2-350}{350T_2}]](/tpl/images/0519/8584/3464f.png)
![- 0.4479 = 41317.599 [\frac{T_2-350}{350T_2} ]K](/tpl/images/0519/8584/1e76b.png)
![-\frac{0.4479}{4137.599} = [\frac{T_2-350}{350T_2} ]](/tpl/images/0519/8584/1ae08.png)
![- 1.0825118*10^{-4} = [\frac{T_2-350}{350T_2} ]](/tpl/images/0519/8584/1627c.png)