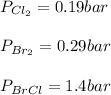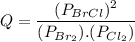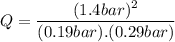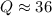Chemistry, 21.02.2020 23:49, Katlynnmarkle13
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If the mixture is analyzed and found to contain 0.19 bar of Cl2, 0.29 bar of Br2 and 1.4 bar of BrCl, describe the situation:a) Q > K and more reactants will be made to reach equilibrium. b) Q > K and more products will be made to reach equilibrium. c) Within 1 decimal place, Q = K and the reaction is at equilibriumd) Q < K and more products will be made to reach equilibrium. e) Q < K and more reactants will be made to reach equilibrium.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1



Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
Do you know the correct answer?
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If...
Questions in other subjects:

Geography, 30.06.2019 15:30

Biology, 30.06.2019 15:30

Mathematics, 30.06.2019 15:30

History, 30.06.2019 15:30

History, 30.06.2019 15:30



Spanish, 30.06.2019 15:30