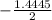Chemistry, 21.02.2020 23:50, westlakebuddy1229
Picric acid has been used in the leather industry and in etching copper. However, its laboratory use has been restricted because it dehydrates on standing and can become shock sensitive. It has an acid dissociation constant of 0.42.
a. What is the [H3O+] for an 0.52 M solution of picric acid? Enter to 4 decimal places.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
Do you know the correct answer?
Picric acid has been used in the leather industry and in etching copper. However, its laboratory use...
Questions in other subjects:


Health, 04.02.2022 08:20



Mathematics, 04.02.2022 08:20

Mathematics, 04.02.2022 08:20

Mathematics, 04.02.2022 08:20

Health, 04.02.2022 08:20

Chemistry, 04.02.2022 08:20

Mathematics, 04.02.2022 08:20


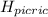
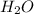 ⇄
⇄ 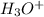 +
+ 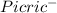
 ) = 0.42
) = 0.42![K_a = \frac{[H_3O^+][Picric^-]}{H_{picric}}](/tpl/images/0519/8296/7a480.png)
![0.42 = \frac{[x][x]}{0.52-x}}](/tpl/images/0519/8296/03f90.png)
![0.42 = \frac{[x]^2}{0.52-x}}](/tpl/images/0519/8296/6158a.png)
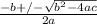 ; ( where +/- represent ± )
; ( where +/- represent ± )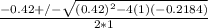
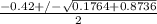
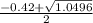 OR
OR 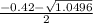
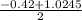 OR
OR 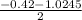
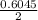 OR
OR