Chemistry, 21.02.2020 21:53, jalenshayewilliams
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order reaction. C2H5I C2H4 + HI At 600 K, the value of k is 1.60 × 10– 5 s– 1. When the temperature is raised to 700 K, the value of k increases to 6.36 × 10– 3 s– 1. What is the activation energy for this reaction?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Do you know the correct answer?
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order...
Questions in other subjects:

Mathematics, 22.04.2020 00:52


Mathematics, 22.04.2020 00:52

Mathematics, 22.04.2020 00:52





Mathematics, 22.04.2020 00:52


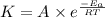
![\log (\frac{K_2}{K_1})=\frac{E_a}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0519/6420/1504e.png)
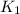 = rate constant at
= rate constant at 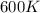 =
= 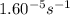
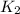 = rate constant at
= rate constant at 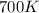 =
= 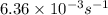
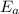 = activation energy for the reaction = ?
= activation energy for the reaction = ?
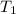 = initial temperature =
= initial temperature = 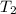 = final temperature =
= final temperature = ![\log (\frac{6.36\times 10^{-3}s^{-1}}{1.60\times 10^{-5}s^{-1}})=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/54c04.png)
![2.60=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/52269.png)