Chemistry, 21.02.2020 21:57, usagimiller
The standard enthalpies of combustion of cis-2-hexene and trans-2-hexene (to form carbon dioxide and water) are −3727.9 kJ·mol−1 and −3726.3 kJ·mol−1, respectively. Calculate the enthalpy of the following isomerization process.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1



Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
Do you know the correct answer?
The standard enthalpies of combustion of cis-2-hexene and trans-2-hexene (to form carbon dioxide and...
Questions in other subjects:

Mathematics, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10


Mathematics, 28.01.2021 06:10


Mathematics, 28.01.2021 06:10

English, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10


Mathematics, 28.01.2021 06:10


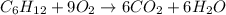
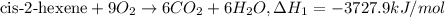 ..[1]
..[1]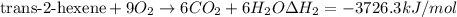 [2]
[2]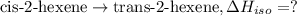 ...[3]
...[3]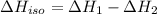 (Hess's law)
(Hess's law)