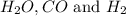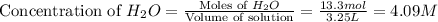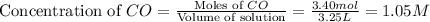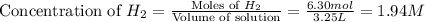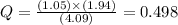The following reaction was carried out in a 3.25 L reaction vessel at 1100 K:
If during...

The following reaction was carried out in a 3.25 L reaction vessel at 1100 K:
If during the course of the reaction, the vessel is found to contain 5.25 mol of C, 13.3 mol of H₂O, 3.40 mol of CO, and 6.30 mol of H₂, what is the reaction quotient Q?
Enter the reaction quotient numerically.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
Do you know the correct answer?
Questions in other subjects:






Mathematics, 06.07.2019 06:00


English, 06.07.2019 06:00



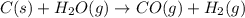
![Q=\frac{[CO][H_2]}{[H_2O]}](/tpl/images/0519/6055/f765a.png)