
Given the equation representing a reaction: H + H ---> H₂What occurs during this reaction? a. A bond is broken and energy is absorbed. b. A bond is broken and energy is released. c. A bond is formed and energy is absorbed. d. A bond is formed and energy is released.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
Do you know the correct answer?
Given the equation representing a reaction: H + H ---> H₂What occurs during this reaction? a. A b...
Questions in other subjects:


Mathematics, 17.09.2021 15:30

Mathematics, 17.09.2021 15:30

Biology, 17.09.2021 15:30



Mathematics, 17.09.2021 15:30

Mathematics, 17.09.2021 15:30

Chemistry, 17.09.2021 15:30

Mathematics, 17.09.2021 15:30


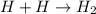 (ΔH = Positive)
(ΔH = Positive)



