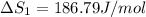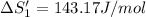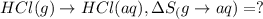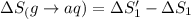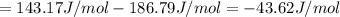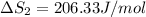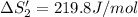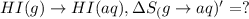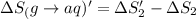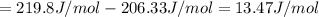All of the hydrogen halides (H-X) are gaseous in their natural state, but dissolve in water to form the aqueous phase.
Entropies (S) for the gaseous H-X molecules (before reaction) are:
HCl (g): 186.79 J/mol
HI (g): 206.33 J/mol
Entropies (S) for the H-X molecules dissolved/solvated in water (after reaction) are:
HCl (aq): 143.17 J/mol
HI (aq): 219.8 J/mol
1. Calculate the ΔS that each of these H-X compounds undergoes as it transitions from the gas phase to the aqueous phase.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, lorenaandreahjimenez
The answer for #3 is c but i don't know why
Answers: 1


Do you know the correct answer?
All of the hydrogen halides (H-X) are gaseous in their natural state, but dissolve in water to form...
Questions in other subjects:

History, 01.08.2019 10:20


Biology, 01.08.2019 10:20

Mathematics, 01.08.2019 10:20

History, 01.08.2019 10:20

Mathematics, 01.08.2019 10:20

History, 01.08.2019 10:20

English, 01.08.2019 10:20

Computers and Technology, 01.08.2019 10:20


![\Delta S=[\text{Sum of entropy of products}]-[\text{Sum of entropy of reactants}]](/tpl/images/0519/3497/4a681.png)