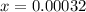Chemistry, 21.02.2020 18:35, michelle8978
Iodine and bromine react to give iodine monobromide, IBr. Ilg) + Br2(g) 2 Br(g) What is the equilibrium composition of a mixture at 145 C that initially contained 0.0019 mol each of iodine and bromine in a 5.0-L vessel? The equilibrium constant K, for this reaction at 145 C is 108.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1



Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
Do you know the correct answer?
Iodine and bromine react to give iodine monobromide, IBr. Ilg) + Br2(g) 2 Br(g) What is the equilibr...
Questions in other subjects:


Mathematics, 14.12.2020 18:30

Biology, 14.12.2020 18:30

Chemistry, 14.12.2020 18:30

Geography, 14.12.2020 18:30

English, 14.12.2020 18:30

Mathematics, 14.12.2020 18:30

History, 14.12.2020 18:30

Geography, 14.12.2020 18:30


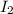 = 0.00006 M
= 0.00006 M 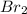 = 0.00006 M
= 0.00006 M 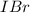 = 0.00064 M
= 0.00064 M 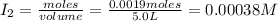
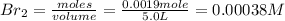
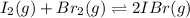
![K_c=\frac{[IBr]^2}{[I_2]\times [Br_2]}](/tpl/images/0519/3166/d47bc.png)
![108=\frac{[2x]^2}{(0.00038-x)^2}](/tpl/images/0519/3166/891f8.png)