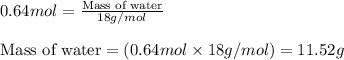Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
Do you know the correct answer?
Trisilane (Si3H8) is a liquid with a density of 0.739 g cm-3. It reacts with oxygen to give silicon...
Questions in other subjects:


Mathematics, 02.07.2019 02:30

Mathematics, 02.07.2019 02:30




History, 02.07.2019 02:30

Biology, 02.07.2019 02:30



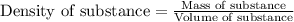
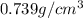
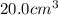
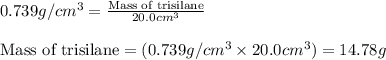
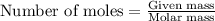 .....(1)
.....(1)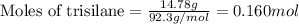
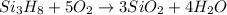
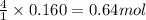 of water
of water