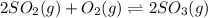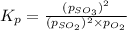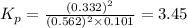Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 06:30, Liapis
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
Do you know the correct answer?
Consider the reaction below 2SO2(g) + O2(g) ⇌ 2SO3(g) At 1000 K the equilibrium pressures of the thr...
Questions in other subjects:

Business, 10.08.2021 01:00





Mathematics, 10.08.2021 01:00

English, 10.08.2021 01:00




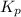 for the reaction is 3.45
for the reaction is 3.45