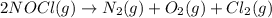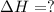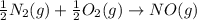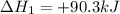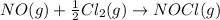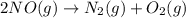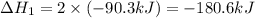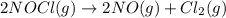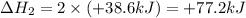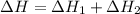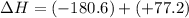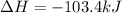Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2



Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
Do you know the correct answer?
N2(g) + O2(g) → NO(g) ΔHrxn = +90.3 kJ NO(g) + Cl2(g) → NOCl(g) ΔHrxn = –38.6 kJ What is the value o...
Questions in other subjects:

Mathematics, 31.08.2019 11:50

Social Studies, 31.08.2019 11:50


Mathematics, 31.08.2019 11:50

Mathematics, 31.08.2019 11:50

Mathematics, 31.08.2019 11:50


Social Studies, 31.08.2019 11:50


Mathematics, 31.08.2019 11:50


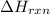 is, -103.4 kJ
is, -103.4 kJ