
Chemistry, 21.02.2020 05:56, swordnewsnetwork
A 71.0 mL 71.0 mL aliquot of a 1.30 M 1.30 M solution is diluted to a total volume of 248 mL. 248 mL. A 124 mL 124 mL portion of that solution is diluted by adding 133 mL 133 mL of water. What is the final concentration? Assume the volumes are additive.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
Do you know the correct answer?
A 71.0 mL 71.0 mL aliquot of a 1.30 M 1.30 M solution is diluted to a total volume of 248 mL. 248 mL...
Questions in other subjects:


History, 06.05.2020 02:22









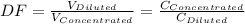
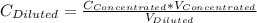 = 1.3×71/248 = 0.372 M
= 1.3×71/248 = 0.372 M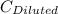 = concentration of new solution
= concentration of new solution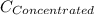 = 0.372 M
= 0.372 M 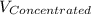 = 124 mL
= 124 mL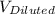 = 257 mL
= 257 mL



