
Chemistry, 21.02.2020 05:28, cjtambasco
"Magnesium has three naturally occurring isotopes: A. Mg-24 with mass 24.3050 amu and a natural abundance of 78.99 %, B. Mg-25 with mass 24.9858 amu and a natural abundance of 10.00 %, and C. Mg-26 with mass 25.9826 amu and a natural abundance of 11.01 %."

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1



Do you know the correct answer?
"Magnesium has three naturally occurring isotopes: A. Mg-24 with mass 24.3050 amu and a natural abun...
Questions in other subjects:




Mathematics, 13.12.2019 01:31



Mathematics, 13.12.2019 01:31

Biology, 13.12.2019 01:31

History, 13.12.2019 01:31

Mathematics, 13.12.2019 01:31


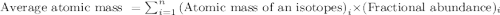 .....(1)
.....(1)![\text{Average atomic mass of magnesium}=[(24.3050\times 0.7899)+(24.9858\times 0.1000)+(25.9826\times 0.1101)]\\\\\text{Average atomic mass of magnesium}=24.5578amu](/tpl/images/0518/9477/add94.png)




