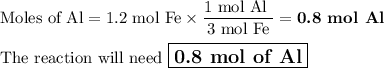Question 21
0.1 pts
Given the following equation:
2 Al(s) +3 FeO(s) 3Fe(s)+ Al2O3(...


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Chemistry, 01.09.2021 06:10

Mathematics, 01.09.2021 06:10

Mathematics, 01.09.2021 06:10



Mathematics, 01.09.2021 06:10