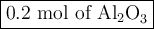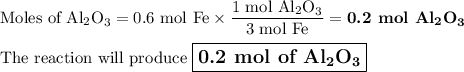Questlon 22
U.1 pts
Given the following equation:
2Al(s) +3 FeO(s)
3Fe(s)+ A...


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2


Do you know the correct answer?
Questions in other subjects:





Biology, 27.07.2019 09:20

Social Studies, 27.07.2019 09:20


Business, 27.07.2019 09:20

History, 27.07.2019 09:20