
Chemistry, 21.02.2020 03:56, reticentrobbie
Calculate the pH of a 0.078 M solution of NaOCl (Ka of HClO = 3.5 x 10-8). Enter pH to 3 decimal places.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Do you know the correct answer?
Calculate the pH of a 0.078 M solution of NaOCl (Ka of HClO = 3.5 x 10-8). Enter pH to 3 decimal pla...
Questions in other subjects:

Mathematics, 24.09.2021 07:40


Mathematics, 24.09.2021 07:40


English, 24.09.2021 07:40


Mathematics, 24.09.2021 07:40

Chemistry, 24.09.2021 07:40



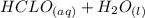 ⇄
⇄ 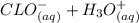
![K_a = \frac{[CLO^-].[H_3O^+]}{[HCLO]} \\\\3.5X10^{-8} = \frac{[x].[x]}{[0.078-x]} \\\\3.5X10^{-8} =\frac{x^2}{0.078-x} \\\\x^2 = 3.5X10^{-8} (0.078-x)\\\\x^2 = 2.73X10^{-9} - (3.5X10^{-8})x\\\\x^2 + (3.5X10^{-8})x - 2.73X10^{-9} =0](/tpl/images/0518/7894/66965.png)




