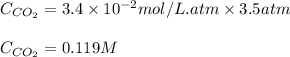Chemistry, 21.02.2020 03:00, Brennen435
The partial pressure of carbon dioxide in a gas mixture is 3.5 atm. What will be the solubility (in M) of carbon dioxide gas when Henry's Law constant for carbon dioxide is 3.4 x 10-2M/atm

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, lilyjordan5972
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Do you know the correct answer?
The partial pressure of carbon dioxide in a gas mixture is 3.5 atm. What will be the solubility (in...
Questions in other subjects:


Mathematics, 15.07.2020 01:01


Mathematics, 15.07.2020 01:01

Physics, 15.07.2020 01:01


Mathematics, 15.07.2020 01:01

English, 15.07.2020 01:01



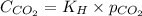
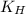 = Henry's constant =
= Henry's constant = 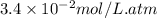
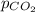 = partial pressure of carbon dioxide gas = 3.5 atm
= partial pressure of carbon dioxide gas = 3.5 atm