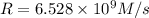Chemistry, 21.02.2020 00:00, ylianafghgfdsnm1479
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way:
OCl−+I−→OI−+Cl−.
This rapid reaction gives the following rate data:
[OCl−](M) [I]−(M) Rate (M/s)
1.5×10^−3 1.5×10^−3 1.36×10^−4
3.0×10^−3 1.5×10^−3 2.72×10^−4
1.5×10^−3 3.0×10^−3 2.72×10^−4
a. Write the rate law for this reaction.
b. Calculate the rate constant with proper units.
c. Calculate the rate when [OCl-]= 1.8×10^3 M and [I-]= 6.0×10^4 M .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2


Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
Do you know the correct answer?
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the foll...
Questions in other subjects:

Biology, 10.03.2021 16:10

Biology, 10.03.2021 16:10

English, 10.03.2021 16:10

Mathematics, 10.03.2021 16:10


Mathematics, 10.03.2021 16:10

Mathematics, 10.03.2021 16:10




![R=k[OCl^-]^1\times [I^-]^1](/tpl/images/0518/3250/d583c.png)
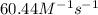 .
.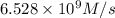 .
.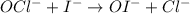
![R=k[OCl^-]^x\times [I^-]^y](/tpl/images/0518/3250/04fdb.png)
![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/e45d8.png) ..[1]
..[1]![[OCl^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/3317d.png) and
and ![2.72\times 10^{-4}M/s=k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/dea0f.png) ..[2]
..[2]![[I^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/b24ad.png) .
.![2.72\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y](/tpl/images/0518/3250/22116.png) ..[3]
..[3]![\frac{1.36\times 10^{-4}M/s}{2.72\times 10^{-4}M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}](/tpl/images/0518/3250/ebd3d.png)
![\frac{1.36\times 10^{-4} M/s}{2.72\times 10^{-4} M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y}](/tpl/images/0518/3250/11fc2.png)
![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]](/tpl/images/0518/3250/f3ed7.png)
![k=\frac{1.36\times 10^{-4} M/s}{[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]}=60.44 M^{-1}s^{-1}](/tpl/images/0518/3250/1ed07.png)
![[OCl^-]=1.8\times 10^{3} M](/tpl/images/0518/3250/b9482.png) and
and ![[I^-]=6.0\times 10^{4} M](/tpl/images/0518/3250/ceced.png) be R.
be R.![R=60.44 M^{-1}s^{-1}\times [1.8\times 10^{3} M]^1\times [6.0\times 10^{4} M]^1](/tpl/images/0518/3250/1384b.png)