Chemistry, 20.02.2020 23:03, NicoleParker
An imaginary element with BCC structure and has an atomic radius of 0.17 nm, with a molar mass of 56.08 g/mol. What is the density of this element in g/cc? hint: you will need Avogadro's number and you will need to convert the given radius to cm.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
Do you know the correct answer?
An imaginary element with BCC structure and has an atomic radius of 0.17 nm, with a molar mass of 56...
Questions in other subjects:

Social Studies, 26.06.2019 23:00




Arts, 26.06.2019 23:00


Mathematics, 26.06.2019 23:00


History, 26.06.2019 23:00

Mathematics, 26.06.2019 23:00


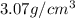
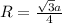
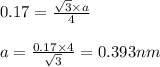
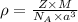
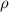 = density
= density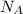 = Avogadro's number =
= Avogadro's number = 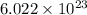
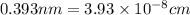 (Conversion factor:
(Conversion factor:  )
)