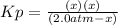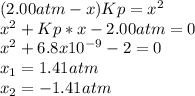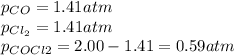Chemistry, 20.02.2020 23:05, transfergiecek8765
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the compounds used to make Lexan is phosgene (COCl2), an extremely poisonous gas. Phosgene decomposes by the following reaction for which Kp = 6.8 ✕ 10-9 at 100°C. COCl2(g) equilibrium reaction arrow CO(g) + Cl2(g) If pure phosgene at an initial pressure of 2.0 atm decomposes, calculate the equilibrium pressures of all species.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
Do you know the correct answer?
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the c...
Questions in other subjects:

Mathematics, 29.10.2020 21:50




History, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50



Biology, 29.10.2020 21:50


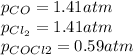
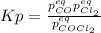
 , the law of mass action becomes:
, the law of mass action becomes: