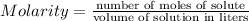Chemistry, 20.02.2020 22:27, mbrisen7420
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of water would you have to add to this solution to reduce the molarity you calculated in Part A (.233 moles) by a factor of two?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1


Chemistry, 23.06.2019 10:30, malum2009
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
Do you know the correct answer?
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of...
Questions in other subjects:



Mathematics, 16.09.2021 08:50


Mathematics, 16.09.2021 08:50


Physics, 16.09.2021 08:50

Social Studies, 16.09.2021 08:50

Computers and Technology, 16.09.2021 08:50