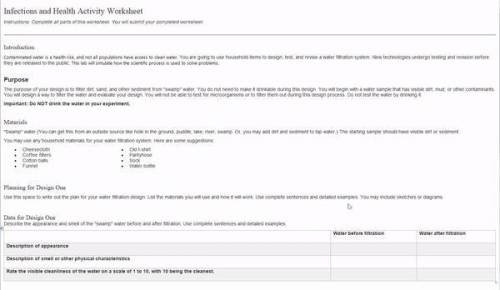
Infections and Health Activity Worksheet
Instructions: Complete all parts of this worksheet. Y...

Chemistry, 20.02.2020 22:32, isiahamccoy8822
Infections and Health Activity Worksheet
Instructions: Complete all parts of this worksheet. You will submit your completed worksheet.
_
Introduction
Contaminated water is a health risk, and not all populations have access to clean water. You are going to use household items to design, test, and revise a water filtration system. New technologies undergo testing and revision before they are released to the public. This lab will simulate how the scientific process is used to solve problems.
Purpose
The purpose of your design is to filter dirt, sand, and other sediment from "swamp" water. You do not need to make it drinkable during this design. You will begin with a water sample that has visible dirt, mud, or other contaminants. You will design a way to filter the water and evaluate your design. You will not be able to test for microorganisms or to filter them out during this design process. Do not test the water by drinking it.
Important: Do NOT drink the water in your experiment.
Materials
"Swamp" water (You can get this from an outside source like hole in the ground, puddle, lake, river, swamp. Or, you may add dirt and sediment to tap water.) The starting sample should have visible dirt or sediment.
You may use any household materials for your water filtration system. Here are some suggestions:
• Cheesecloth
• Coffee filters
• Cotton balls
• Funnel • Old t-shirt
• Pantyhose
• Sock
• Water bottle
Planning for Design One
Use this space to write out the plan for your water filtration design. List the materials you will use and how it will work. Use complete sentences and detailed examples. You may include sketches or diagrams.
Data for Design One
Describe the appearance and smell of the "swamp" water before and after filtration. Use complete sentences and detailed examples.
Water before filtration Water after filtration
Description of appearance
Description of smell or other physical characteristics
Rate the visible cleanliness of the water on a scale of 1 to 10, with 10 being the cleanest.
Feedback
Consult a peer or a family member about ways your design could be improved. Describe the feedback and provide the name of the reviewer. These are only suggestions that you can use to plan for Design Two. Use complete sentences and detailed examples.
Planning for Design Two
Use this space to plan out how to improve your water filter. Based on the feedback you received, what part are you trying to improve? Why did you make the changes that you did? Use complete sentences and detailed examples. You may include sketches or diagrams.
Data for Design Two
Use the data and feedback from your first design to make changes to your water filtration system. Test your new design. Record the appearance and smell of the "swamp" water before and after filtration. Use complete sentences and detailed examples.
Water before filtration Water after filtration
Description of appearance
Description of smell or other physical characteristics
Rate the visible cleanliness of the water on a scale of 1 to 10, with 10 being the cleanest.
Conclusion
Use your data from Design One and Design Two to answer the following questions. Use complete sentences and detailed examples.
1. Summarize the appearance and smell of the "swamp" water before and after the filtering process using Design One.
2. Describe the changes you made to your water filtration system.
3. What feedback helped you make changes to your water filtration system?
4. Summarize the appearance and smell of the "swamp" water before and after the filtering process using Design Two.
5. What did you learn about the process of planning, testing, and revising a design?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 26.05.2021 18:30


Mathematics, 26.05.2021 18:30

Advanced Placement (AP), 26.05.2021 18:30





Mathematics, 26.05.2021 18:30






