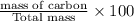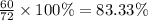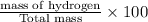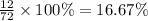Chemistry, 20.02.2020 08:29, ARAYAMYHAND
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: C5H12. Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. View Available Hint(s)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
Do you know the correct answer?
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composit...
Questions in other subjects:


Mathematics, 25.02.2021 01:00


Chemistry, 25.02.2021 01:00

Social Studies, 25.02.2021 01:00






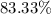 and
and 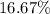 respectively.
respectively.