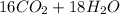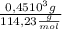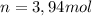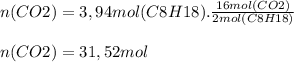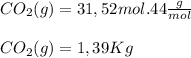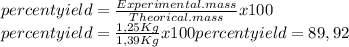Chemistry, 20.02.2020 08:12, hebrew1148
0.450 kg of octane burned during combustion produces 1.25 kg of carbon dioxide. what is the percent yield?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1
Do you know the correct answer?
0.450 kg of octane burned during combustion produces 1.25 kg of carbon dioxide. what is the percent...
Questions in other subjects:


Mathematics, 03.12.2020 06:10



History, 03.12.2020 06:10

History, 03.12.2020 06:10

Health, 03.12.2020 06:10



Mathematics, 03.12.2020 06:10


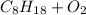 →
→ 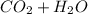
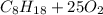 →
→